Contact: +91 99725 24322 |
Menu
Menu
Quick summary: Explore how EUDR compliance impacts the medical industry, ensuring sustainable, deforestation-free sourcing of materials like natural rubber. Learn how healthcare companies can navigate these regulations to protect the environment and maintain EU market access.

The world is waking up to the urgent need to protect our forests and mitigate the devastating effects of deforestation. Amid this global shift, the EU Deforestation Regulation (EUDR) has emerged as a powerful tool in the fight against deforestation. At first glance, the connection between medical products and deforestation might seem tenuous. However, dig a little deeper, and the link becomes clear. Natural rubber, a critical component in medical products such as gloves, catheters, and tubing, is often sourced from regions where deforestation is rampant. This mandates EUDR Compliance for Healthcare Sector
The EUDR places the onus on manufacturers to ensure that their supply chains are free from deforestation, demanding transparency and accountability at every step. But why should medical product manufacturers care? Beyond the ethical implications, the stakes are high. Non-compliance with the EUDR can lead to significant financial penalties, legal risks, and, perhaps most damaging of all, a loss of trust among consumers and partners. In an industry where safety, reliability, and ethics are paramount, aligning with the EUDR is not just about following the rules—it’s about securing your reputation and future.
The EUDR is more than just a set of rules; it’s a bold step towards a sustainable future. Designed to curb deforestation driven by the production of key commodities, this regulation mandates strict due diligence for businesses involved in the import, export, and trading of products linked to deforestation. The scope of the EUDR is broad, covering everything from soy and palm oil to rubber and wood. For industries like agriculture, food, and even healthcare, this regulation is not just a guideline—it’s a game changer.
As the EU Deforestation Regulation (EUDR) tightens its grip on global trade, industries far and wide are feeling the impact—none more so than the healthcare sector. While the connection between healthcare and deforestation might not be immediately obvious, the reality is that many essential healthcare products are directly linked to raw materials like natural rubber, which is often sourced from areas prone to deforestation. Understanding the EUDR requirements is not just about ticking boxes; it’s about ensuring your business remains on the right side of this critical regulation. Ensuring natural rubber compliance under EUDR is essential for maintaining market access and demonstrating a commitment to sustainable, deforestation-free sourcing.
The EUDR casts a wide net, and if your business deals with medical gloves, latex products, rubber tubing, or similar items, you’re squarely in its sights. These products, often made from natural rubber, are staples in the Healthcare industry, used daily in hospitals, clinics, and labs worldwide. The challenge is that the rubber used in these products is often sourced from regions where deforestation is a significant issue. Latex, a vital raw material for medical products, presents unique challenges within the healthcare supply chain. Ensuring sustainable and ethical sourcing of latex is crucial for the production of essential items like gloves and catheters.
1. Medical Gloves
2. Latex Products
3. Rubber Tubing and Components
4. Disposable Medical Items
5. Rubber Parts in Medical Devices
6. Other Natural Rubber Products
For manufacturers and suppliers, this means a thorough examination of where and how these materials are obtained. It’s not enough to know that the rubber came from a reputable supplier—you need to trace its origins back to the very trees it was harvested from. This level of detail is crucial for complying with EUDR, which demands transparency and proof that the products you’re bringing into the EU market are deforestation-free.
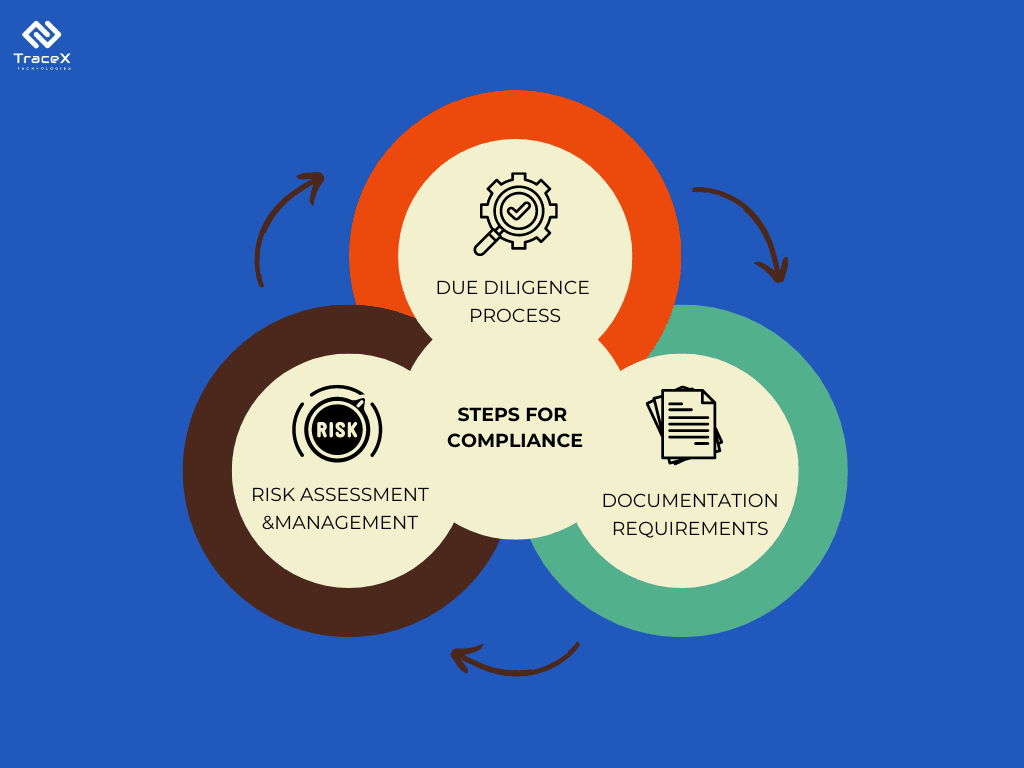
So, what does compliance actually involve? The EUDR isn’t just asking for good intentions; it’s requiring hard evidence. For each product type—whether it’s medical gloves, latex-based products, or rubber tubing—you’ll need to provide comprehensive documentation for due diligence. This includes detailed records of the supply chain, proof of sustainable sourcing practices, and verification that no deforestation occurred in the production of the raw materials.
Meeting these criteria isn’t just a regulatory necessity—it’s a way to build trust with your customers and stakeholders, showing them that you’re committed to sustainability and ethical practices.
One of the critical aspects of the EUDR is its broad geographic scope. It doesn’t matter whether your suppliers are based in the EU or halfway around the world; if you’re placing products on the EU market, the regulation applies to you. This global reach means that even if you’re sourcing rubber from a plantation in Southeast Asia or South America, you’re still responsible for ensuring that your products meet EUDR standards.
For international suppliers, this can be particularly challenging, as it requires close collaboration with partners who might not be familiar with the regulation’s demands. However, this also presents an opportunity to strengthen relationships with your suppliers by working together to ensure compliance.
The EUDR is a wake-up call for the entire supply chain to operate more transparently and sustainably, and those who rise to the challenge will not only avoid penalties but also gain a competitive edge in the market.
For industries reliant on natural resources, like the healthcare sector, consumers and regulators alike demand accountability and traceability solutions has never been more critical. The EU Deforestation Regulation (EUDR) underscores the importance of knowing exactly where and how your products are sourced. But achieving this level of transparency is easier said than done.
Traceability is the backbone of EUDR compliance. For medical products like gloves, latex, and rubber tubing, ensuring that your raw materials are deforestation-free means having a clear, unbroken chain of custody from the source to the end product. Traceability allows companies to verify the origin of their materials and prove that they haven’t contributed to deforestation—an absolute necessity under the EUDR.
Without robust traceability, you’re left vulnerable to supply chain disruptions, reputational damage, and hefty fines. But beyond the regulatory aspect, traceability builds trust with your customers.
It’s a way of saying, “We care about where our products come from, and we’re committed to protecting the environment.” In an age where consumers are more environmentally conscious than ever, this can set your brand apart.
So, how do you achieve this level of transparency? Technology offers powerful tools to help businesses track and verify their supply chains. Blockchain technology, for example, is becoming a game-changer in traceability. By creating an immutable ledger of transactions, blockchain ensures that every step in the supply chain is recorded and verifiable. This means that once the raw materials for your healthcare products enter the supply chain, you can trace them every step of the way, right through to the finished product.
Satellite monitoring is another cutting-edge solution. With the ability to track land use changes in real-time, satellites can provide direct evidence of whether deforestation has occurred in areas where your raw materials are sourced. This kind of data is invaluable when proving compliance with the EUDR.
The TraceX EUDR Compliance Platform is designed to help businesses navigate the complex requirements of the EU Deforestation Regulation (EUDR) with ease and confidence. This advanced platform leverages blockchain technology to ensure transparency, traceability, and accountability across the entire supply chain. It allows companies to track the origin of their raw materials, verify deforestation-free status, and maintain detailed records that meet EUDR’s stringent due diligence standards. Additionally, the platform integrates satellite monitoring solutions for real-time deforestation risk assessments and mitigation, enabling businesses to proactively address potential compliance issues and ensure their supply chains remain sustainable. By using TraceX, businesses can streamline compliance processes, mitigate risks, and demonstrate their commitment to sustainable practices, ultimately positioning themselves as responsible players in the global market.
The EU Deforestation Regulation (EUDR) is set to have a profound impact on global supply chains, particularly for international suppliers. As the regulation demands stringent due diligence and proof of deforestation-free sourcing, suppliers around the world are feeling the pressure to meet these new standards. This shift is not just a regulatory burden but a significant change in how global trade operates. Suppliers from regions where deforestation risks are high, such as Southeast Asia and South America, are particularly affected. They now face the challenge of providing verifiable evidence that their products, whether raw materials like palm oil or rubber, or finished goods, are not contributing to deforestation. Failure to comply could result in losing access to the lucrative EU market, making EUDR a critical factor in global trade relations.
For suppliers outside the EU, the path to compliance may seem daunting, but it is navigable with the right strategies. The first step is understanding the specific requirements of the EUDR and how they apply to each product category. This means not only conducting thorough due diligence but also adopting advanced traceability technologies that can track products from their origin to their final destination. Blockchain, for example, can offer an immutable record of transactions, ensuring transparency throughout the supply chain. Non-EU suppliers should also consider partnerships with EU-based companies or certification bodies that are familiar with the regulation. These collaborations can help streamline the compliance process and provide the necessary documentation to meet EUDR standards. By taking proactive measures, non-EU suppliers can not only comply with the regulation but also strengthen their position in the global market, turning compliance into a competitive advantage.
EUDR compliance doesn’t exist in a vacuum; it intersects with other regulations that govern the medical industry. For instance, healthcare companies are already subject to strict standards for product safety, quality, and environmental impact. The introduction of EUDR adds another layer of regulatory complexity, requiring companies to navigate overlapping rules and ensure comprehensive compliance. This might involve integrating EUDR requirements with existing environmental, social, and governance (ESG) frameworks, or aligning with global standards like ISO 14001 for environmental management. Understanding and managing these overlaps is crucial for healthcare companies aiming to stay compliant and competitive in a rapidly evolving regulatory landscape.
Navigating EUDR compliance in the healthcare industry is not just a regulatory necessity—it’s a commitment to sustainability and ethical sourcing. By adhering to these regulations, companies in the healthcare sector can ensure that their supply chains are free from deforestation, protecting both the environment and the integrity of their products. Embracing EUDR compliance will not only safeguard market access in the EU but also enhance corporate reputation and align with global sustainability goals. As the medical industry continues to innovate and grow, integrating these compliance measures will be crucial in shaping a responsible and resilient future.